 Cells are made up of a wide range of complex molecules, called biological macromolecules, like carbohydrates, lipids, proteins and nucleic acids (which I will deal with in my next posts). In this post we will discuss carbon, the basic element of all these macromolecules.
Cells are made up of a wide range of complex molecules, called biological macromolecules, like carbohydrates, lipids, proteins and nucleic acids (which I will deal with in my next posts). In this post we will discuss carbon, the basic element of all these macromolecules.
Carbon (C), atomic number 6 (6 protons and 6 electrons), has an incomplete outermost shell with four electrons in it. Thus, with these four unpaired electrons, it can form up to four covalent bonds with other atoms satisfying, in this way, the octet rule. This property allows carbon to become a really versatile chemical element, ideal as a structural component or as the backbone in macromolecules.
Hydrocarbons
Hydrocarbons are organic molecules[1] completely made up of carbon and hydrogen. The covalent bonds that are formed between the atoms of these molecules release a great amount of energy when they are burnt (oxidized). This is the reason why hydrocarbons are used as fuels in our daily life. Some examples include butane and propane gases.
Each covalent bond between carbon atoms can be simple, double or triple, which influences the geometry and global shape of the molecule, which in turn, influences the properties and functions of hydrocarbons in a basic way. Thus, we find that carbon atoms with simple bonds constitute tetrahedral shapes, which allows the rotation of the molecule along the axis of the bond. However, when double and triple bonds appear, the molecule configuration is planar and linear respectively.
▣ Aliphatic hydrocarbons (hydrocarbon chains): they are made up of successive bonds between carbon atoms which can be branched or unbranched.
▣ Aromatic hydrocarbons (hydrocarbon rings): they are formed by closed rings of five or six carbon atoms. These kinds of structures occur, at times, in double bond hydrocarbons, like cyclohexane or benzene. Benzene has vital importance in some amino acids, like the cholesterol molecule or its derivatives (testosterone and estrogen hormones).
Nevertheless, this division is not definitive because some hydrocarbons present their structure with both aliphatic an aromatic fragments, an example is the beta carotene molecule, which is a powerful antioxidant and precursor of A vitamin, present in fruits, vegetables and grains.
▣ Structural isomers: they differ on account of the situation of their covalent bonds. Thus, butane and isobutane are constituted by 4 carbon atoms and 10 hydrogen atoms each (C4H10), but the different placement of the atoms within the molecule leads to different chemical properties. Whereas the first one (butane) is used as a fuel, the second one is more suitable to use as a refrigerant and a propellant in sprays.
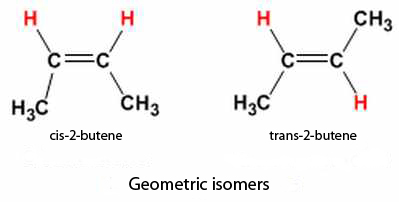
▣ Geometric isomers: they are differentiated by how the atoms are configured around double carbon bonds C=C. Thus, we would talk about cis configuration, when we find the same groups of atoms at the same side of the double bond, and trans configuration, when they are arranged on opposite sides. Trans configuration generates an approximate linear molecular structure, whereas the cis configuration originates a bend (change in direction) of the backbone.
Hydrocarbons are divided, mainly, into two categories:
▣ Aliphatic hydrocarbons (hydrocarbon chains): they are made up of successive bonds between carbon atoms which can be branched or unbranched.
▣ Aromatic hydrocarbons (hydrocarbon rings): they are formed by closed rings of five or six carbon atoms. These kinds of structures occur, at times, in double bond hydrocarbons, like cyclohexane or benzene. Benzene has vital importance in some amino acids, like the cholesterol molecule or its derivatives (testosterone and estrogen hormones).
Isomers
Isomers are molecules that share the same chemical formula but with a different structure or placement of their atoms and/or chemical bonds. We can distinguish two types:
▣ Structural isomers: they differ on account of the situation of their covalent bonds. Thus, butane and isobutane are constituted by 4 carbon atoms and 10 hydrogen atoms each (C4H10), but the different placement of the atoms within the molecule leads to different chemical properties. Whereas the first one (butane) is used as a fuel, the second one is more suitable to use as a refrigerant and a propellant in sprays.
▣ Geometric isomers: they are differentiated by how the atoms are configured around double carbon bonds C=C. Thus, we would talk about cis configuration, when we find the same groups of atoms at the same side of the double bond, and trans configuration, when they are arranged on opposite sides. Trans configuration generates an approximate linear molecular structure, whereas the cis configuration originates a bend (change in direction) of the backbone.
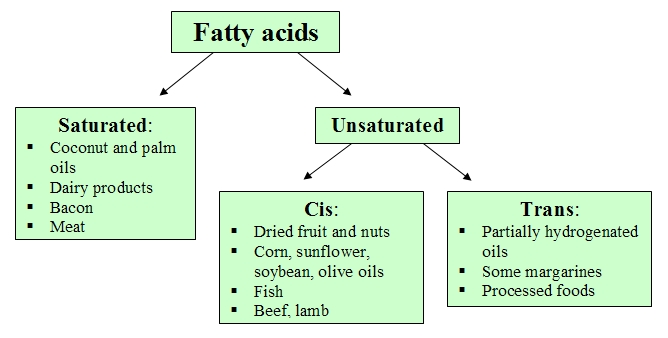
Triglycerides[2] (fats and oils) are classified according to the fatty acids[3] they contain. Thus, those fatty acids with at least one double bond between carbon atoms are unsaturated fats. When some of these bonds appears with cis configuration, the triglycerides molecules cannot be grouped or packed, so they are liquid at room temperature constituting those substances known as oils.
However, if the double bond presents trans configuration, the molecules are able to pack tightly at room temperature forming solid fats, popularly known as trans fats, such as partially hydrogenated oils, processed foods and some margarines. In the human diet, these fats are associated with an increase in cardiovascular diseases, therefore they have been significantly reduced and/or eliminated in lots of food products in food sector.
In contrast, those triglycerides that lack double bonds between carbon atoms are named saturated fats, meaning that they contain all the hydrogen atoms available. These fats usually solidify at room temperature and are found in food of animal origin, milk and its derivates, and even in some oils of plant origin, such as coconut and palm oils.
Enantiomers
Enantiomers are molecules with the same bonds and identical chemical structure but with different arrangement of their atoms, such that they are mirror images of each other. An example is some D and L- forms of amino acids which have very different functions, the D-form of amino acids composes the cell walls in some bacteria, and the L-form of amino acid makes proteins.
Functional groups
Functional groups are groups of atoms in molecules, which give them some specific chemical properties. In this way, each one of the four groups of biological macromolecules (carbohydrates, lipids, proteins and nucleic acids) has its own set of functional groups which provide them chemical properties and very particular functions in living organisms.
These groups are attached to the carbon backbone at several points in macromolecules along their linear chain and/or ring structure.
Functional groups are usually classified on the basis of their charge or polarity, in hydrophobic (uncharged molecules that do not interact well with polar molecules like water) and hydrophilic (polar ions or molecules that do interact well with other polar molecules).
These groups are attached to the carbon backbone at several points in macromolecules along their linear chain and/or ring structure.
Functional groups are usually classified on the basis of their charge or polarity, in hydrophobic (uncharged molecules that do not interact well with polar molecules like water) and hydrophilic (polar ions or molecules that do interact well with other polar molecules).
[1] Those molecules that contain any form of carbon (solid, liquid or gas) which are vital for life.
[2] Compound made of a glycerol molecule as a backbone with three fatty acids attached.
[3] Long aliphatic chains usually containing an even number of carbon atoms (16 - 22). Their structure is mainly hydrophobic (repels water) with a carboxylic group as a functional group with acidic character.
[2] Compound made of a glycerol molecule as a backbone with three fatty acids attached.
[3] Long aliphatic chains usually containing an even number of carbon atoms (16 - 22). Their structure is mainly hydrophobic (repels water) with a carboxylic group as a functional group with acidic character.
Sources: OpenStax College, Biology. OpenStax College. 30 May 2013.
http://perdergrasa.bligoo.es/clasificacion-de-trigliceridos
http://recursos.cnice.mec.es/biosfera/alumno/2bachillerato/biomol/contenidos9.htm
http://biomodel.uah.es/model2/lip/acgr-salud.htm
http://perdergrasa.bligoo.es/clasificacion-de-trigliceridos
http://recursos.cnice.mec.es/biosfera/alumno/2bachillerato/biomol/contenidos9.htm
http://biomodel.uah.es/model2/lip/acgr-salud.htm






Yes, that’s the way I always wanted to come over such a wonderful platform where I could satisfy myself regarding my issues. I found answers of all most of my check list I prepared after having a lot of confusion. Great job.
ReplyDeleteลงทุน forex
This comment has been removed by a blog administrator.
ReplyDeleteThank you very much for your kind comments.
DeleteI?m impressed, I must say. Rarely do I come across a blog that?s equally educative and entertaining, and without a doubt, you have hit the nail on the head. The problem is something that too few folks are speaking intelligently about. Now i'm very happy that I stumbled across this during my hunt for something concerning this.
ReplyDeletetủ gỗ đựng quần áo
That's very kind of you. Glad you like it.
DeleteEveryone loves it whenever people come together and share opinions. Great blog, stick with it!
ReplyDeletegiường đẹp