It is very likely that the term nanomedicine has caught your eye and maybe this is the first time you have heard of it. As nanomedicine can be defined as the application of nanotechnology in the medical field, I will begin this section with a brief view about what nanotechnology is. I will show you the unique features of technologies and materials at nanoscale and a classification of the most common types of structures used in nanomedicine.
But first of all, what is a nanometre?, the prefix “nano” comes from the Greek νάνος, which means “dwarf”. Nowadays, it indicates the billionth part or, in other words, it is a factor of 10-9, therefore, a nanometre (nm) is one billionth of a meter. This way, we can conclude that nanoscience/nanotechnology is the science/engineering that studies and operates with matter in a size from 1 to 100 nm.
The idea and concept of nanotechnology arose from a Richard Feynman’s conference at UCLA University in 1967, where this theoretical physicist introduced, for the first time, the possibility of manipulating atoms and molecules.
But nanotechnology age really began in 1981 when the scanning tunneling microscope (STM) was developed with which we are capable of observing atoms.
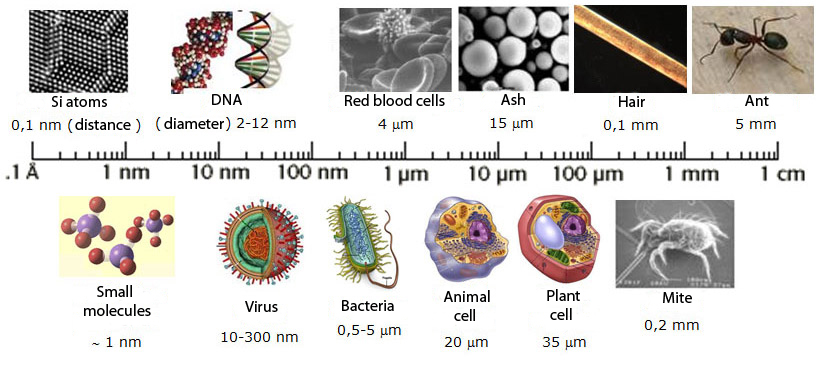
The smallest objects we can observe with our naked eye have a size around a millimetre (the thousandth part of a meter), e.g.: the edge of a coin or a grain of sand, below this magnitude we find it difficult to distinguish objects.
If we divide a millimetre into a thousand parts we are in the micrometer scale, which is the domain of bacteria (5-20 μm) or blood cells (red blood cells: 6-10 μm). Therefore, to observe them we need the help of an optic microscope.
If we keep decreasing the scale and we cut up a micrometer into a thousand parts, we achieve our goal: the nanometre, as I said previously the billionth part of a meter. At this scale we find viruses (20-250 nm) and the DNA molecule (around 2 nm wide).
However, to be able to observe an atom we should still decrease one order of magnitude our scale, as atoms have magnitudes from 0.1 to 0.3 nm.
But nanotechnology age really began in 1981 when the scanning tunneling microscope (STM) was developed with which we are capable of observing atoms.
The smallest objects we can observe with our naked eye have a size around a millimetre (the thousandth part of a meter), e.g.: the edge of a coin or a grain of sand, below this magnitude we find it difficult to distinguish objects.
If we divide a millimetre into a thousand parts we are in the micrometer scale, which is the domain of bacteria (5-20 μm) or blood cells (red blood cells: 6-10 μm). Therefore, to observe them we need the help of an optic microscope.
If we keep decreasing the scale and we cut up a micrometer into a thousand parts, we achieve our goal: the nanometre, as I said previously the billionth part of a meter. At this scale we find viruses (20-250 nm) and the DNA molecule (around 2 nm wide).
However, to be able to observe an atom we should still decrease one order of magnitude our scale, as atoms have magnitudes from 0.1 to 0.3 nm.
To get a more accurate idea about the real size of a nanometre, here are a couple of objects from our daily life measured in nanometres: a human hair is about 50,000-100,000 nm in diameter and a paper sheet is around 100,000 nm thick. Now, let’s do it the other way round, imagine you are shrunk until 10 nm, at that scale a human hair is like the island of Manhattan, a red blood cell like a football stadium, a polio virus like a basketball hoop and a hydrogen atom like a ping-pong ball…...¿surprising?
Right now, you may be wondering what the point is of using such tiny scales and you can find the answer, for instance, in your mobile phone. Miniaturisation has transform the huge, old mobile phones into small computers (with GPS, Internet connection, digital camera…) that you can carry in your pocket.
Nanotechnology has also enabled several breakthroughs like remote medical diagnostic devices, holograms, flexible and 3D screens, or seamless voice control devices.
Therefore, miniaturisation has allowed to locate millions of electronic devices in an area of just a few millimetres.
Nanotechnology has also enabled several breakthroughs like remote medical diagnostic devices, holograms, flexible and 3D screens, or seamless voice control devices.
Therefore, miniaturisation has allowed to locate millions of electronic devices in an area of just a few millimetres.
Surface area to volume ratio
One of the most important properties at nanoscale is the surface-volume ratio. This ratio is an essential parameter in miniaturization and nanotechnology, which states that this ratio increases as we decrease the dimensions of an object and vice versa.
As a material size diminishes, most of its atoms are located on the surface. Let’s consider a 10 nm silicon[1] cube, if we make some calculations we find that it contains around 50,000 atoms in all, of which 680 are located on each face of the cube. So, the overall number of atoms on the surface, multiplying by six, comes to 4,080. Now, if we divide this number by the total amount (50,000 atoms) we obtain that around 8% of the atoms are on the cube surface.Let’s carry out the same calculations with a 10 cm2 and 1 μm thick cube, we get, in this case, that only 0.03% are found on the surface.
Therefore, from these calculations we can conclude that nanomaterials have a greater surface area per unit volume than larger materials. This ratio leads us to a really interesting property in materials at nanoscale: they are much more reactive from a chemical point of view, so they can catalyse reactions more easily, why? because atoms and molecules on surfaces do not have full allocation of covalent bonds, consequently, they are energetically unstable what makes them be more reactive than the non-nanoscale materials.
Because their specific physicochemical properties, nanomaterials have innumerable applications since they can participate in biological processes interacting with biological macromolecules (such as carbohydrates, nucleic acids, lipids and proteins). Also with ions, minerals, water in desalination treatments or even in drug delivery on which particularly I will focus on later.
Hence, there is a paradigm shift in nanotechnology: what is important about materials is not really what they are made of, but how small they are.
Hence, there is a paradigm shift in nanotechnology: what is important about materials is not really what they are made of, but how small they are.
[1] Compound of oxygen and silicon ordered in a three-dimensional structure forming quartz and its types.
Sources: Introduction to Nanotechnology, Prof. Hossam Haick, Israel Institute of Technology.
Rice University. Nanotechnology: The basics.
http://www.quimicaviva.qb.fcen.uba.ar/v11n3/castro.html
Rice University. Nanotechnology: The basics.
http://www.quimicaviva.qb.fcen.uba.ar/v11n3/castro.html






Your opinion matters